In cell and gene therapy, from early clinical development to successful IND/CTA application, our customers around the world trust Miltenyi Bioindustry as their partner in rolling out new therapies. So can you. With our expertise, breakthrough platforms, and tailored service offerings, Miltenyi Bioindustry helps from early clinical trials to large-scale global operations.
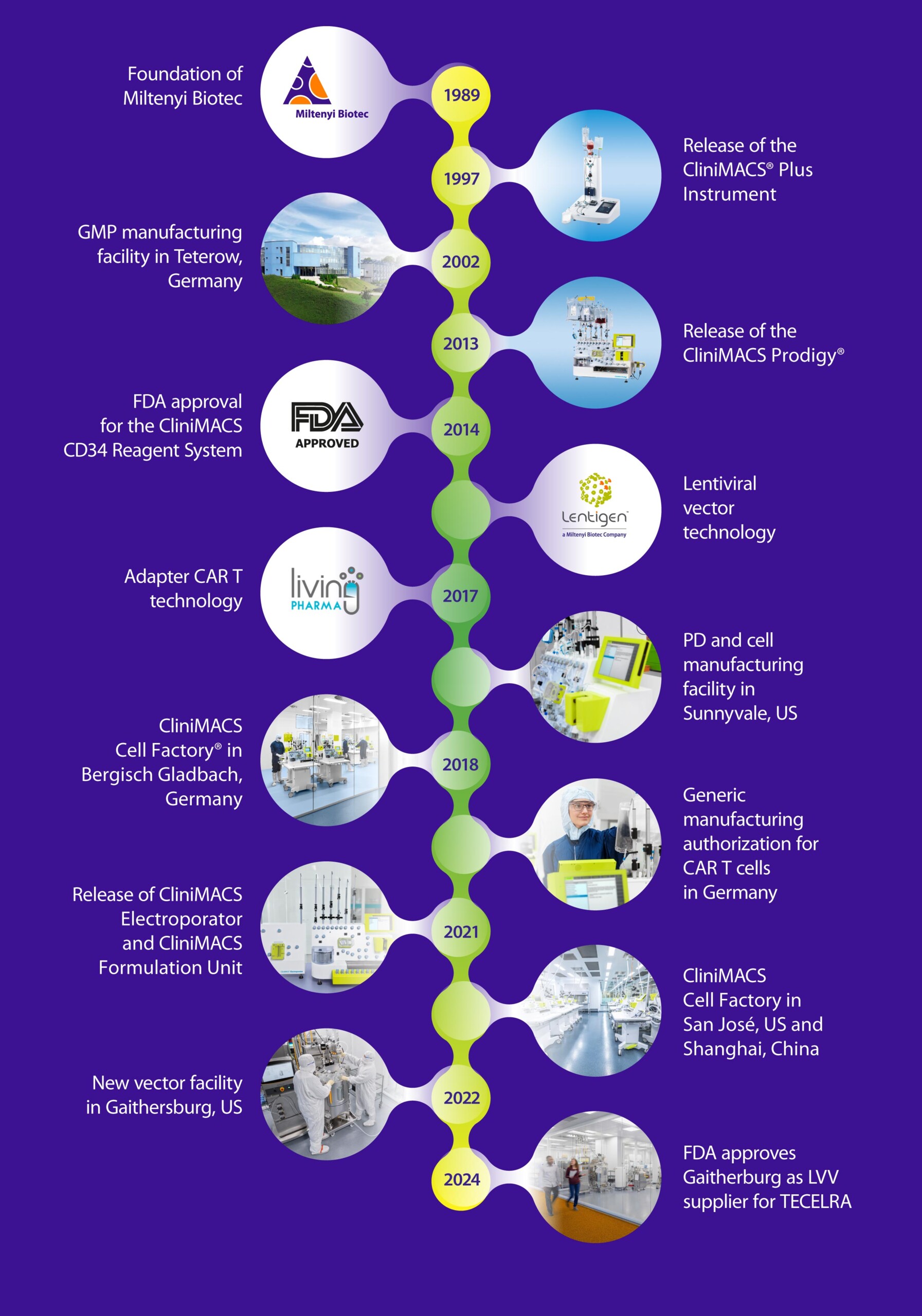
Our commitment to making new therapies a reality began with the development of the CliniMACS® Plus Instrument, the world’s first automated cell separation device, and led to the FDA approval of the CliniMACS® CD34 Reagent System. The launch of the fully automated CliniMACS Prodigy® Platform set the standard for automated, scalable, and GMP-compliant cell processing and manufacturing. The latest addition of the associated CliniMACS® Electroporator and CliniMACS® Formulation Unit modules pushes this standard still further and opens up entirely new possibilities for true and fully integrated end-to-end solutions in cell and gene therapy manufacturing. With this, Miltenyi Bioindustry is able to offer an all-inclusive platform and service package to add true value to your therapy products.
With an emphasis on immunotherapy, regenerative medicine, and graft engineering, Miltenyi Biotec is at the forefront of pioneering clinical research in cell and gene therapy. Our acquisition and integration of the lentiviral vector manufacturing business from Lentigen, and Living Pharma’s CAR T cell therapy technology, has strengthened this still further, allowing us to offer lentiviral vector and cell manufacturing services on a larger scale.
We develop, optimize, and engineer robust, GMP-compliant cell manufacturing processes that are specifically tailored to our customers’ cell therapy needs. With our CliniMACS Cell Factory® Facilities, now available on three continents, we provide standardized and consistent processes tailored to regional requirements and challenges. We are known for our unparalleled commitment to innovation and technical excellence, drawing on our own time-tested technologies and resources.

Around the globe, Miltenyi Biotec products and know-how are in use in trailblazing labs, clinics, and manufacturing sites. Our technologies help to realize more than 100,000 cell therapy procedures, hundreds of clinical trials, and treatments for over 6,000 patients annually. Having passed numerous audits, all our customers trust Miltenyi Biotec to deliver consistently high-quality products with a solid regulatory track record. Working with us means tailored consulting and innovative technologies as the foundation of our full-service CDMO offerings.
As our successful clients can confirm, we are a strong partner with the mission to quickly move you through development and scale-up challenges, right through to successful IND/CTA and towards BLA/MAA submission. We can back up that claim with decades of experience in lentiviral vector design, process development, and GMP-compliant manufacturing, as well as in cell processing and clinical cell therapy manufacturing. With Miltenyi Bioindustry, these capabilities are leveraged by a highly skilled and motivated team of process and analytical experts to provide customized and commercially viable solutions. Reach out to us and let your cell and gene therapy vision become reality.
Humanitarian Device
Authorized by U.S. Federal Law for use in the treatment of patients with acute myeloid leukemia (AML) in first complete remission. The effectiveness of the device for this use has not been demonstrated.
Indications for Use
The CliniMACS® CD34 Reagent System is indicated for processing hematopoietic progenitor cells collected by apheresis (HPC, Apheresis) from an allogeneic, HLA-identical, sibling donor to obtain a CD34+ cell-enriched population for hematopoietic reconstitution following a myeloablative preparative regimen without the need for additional graft versus host disease (GVHD) prophylaxis in patients with acute myeloid leukemia (AML) in first morphologic complete remission.
Contraindications
Do not use CD34+ cells prepared with CliniMACS CD34 Reagent System in patients with known hypersensitivity to murine (mouse) proteins or iron dextran.
Rx only